

Answers: 3
Other questions on the subject: Chemistry



Chemistry, 23.06.2019 06:00, kristine2424
How does a coronal mass ejection (cme) affect the solar wind? a cme adds more particles to the solar wind, intensifying it. a cme blocks the solar wind, causing it to fade. a cme does not affect the solar wind but it does affect auroras. a cme increases the amount of energy in the solar wind.
Answers: 2
Do you know the correct answer?
The bond enthalpy of the oxygen-oxygen bond in O2 is 498 kJ/mol. Based on the enthalpy of the reacti...
Questions in other subjects:







Mathematics, 06.05.2020 21:20

Social Studies, 06.05.2020 21:20



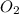 is 498 kJ / mol. What is the average O=O bond energy of the bent ozone molecule O=O=O?
is 498 kJ / mol. What is the average O=O bond energy of the bent ozone molecule O=O=O?
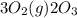
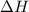 for 2 moles of ozone is
for 2 moles of ozone is 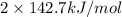 = 285.4 kJ/mol.
= 285.4 kJ/mol.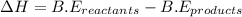
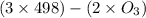
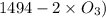
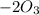
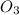 = 604.3 kJ/mol
= 604.3 kJ/mol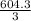 kJ/mol
kJ/mol



