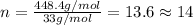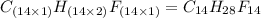Compound consists of carbon, hydrogen and fluorine. In one experiment, combustion of 2.50 g of the compound produced 3.926 g of CO2. Another sample weighing 5.00 g was found to contain 2.54 g of fluorine. The molar mass is found to be 448.4 g/mol. What are its empirical and molecular formulas? (AW(amu): C = 12.01, H = 1.008, F = 19.00)

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:00, parisaidan366
What happened in 2012 and how does it illustrate the importance of understanding the sun and how it works?
Answers: 3

Chemistry, 22.06.2019 11:40, tatemelliott
Calculate the number of kilojoules to warm 125 g of iron from 23.5°c to 78.0°c.
Answers: 3

Do you know the correct answer?
Compound consists of carbon, hydrogen and fluorine. In one experiment, combustion of 2.50 g of the c...
Questions in other subjects:

Arts, 06.05.2021 17:10


English, 06.05.2021 17:10

World Languages, 06.05.2021 17:10







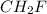 and
and 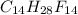 respectively
respectively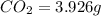
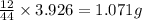 of carbon will be contained.
of carbon will be contained.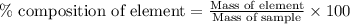 ......(1)
......(1)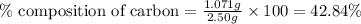
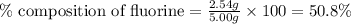
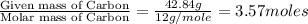
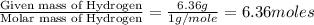
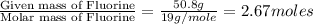
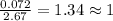
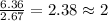
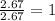
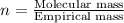
![12+(2\times 1)+19]=33g/mol](/tpl/images/0516/0009/ccdc5.png)