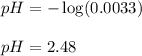Answers: 3
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 00:00, 2024daisjavien
Which actions would increase the rate at salt dissolves in water? stir the water? crush the salt? use less water? heat the water? cool the salt
Answers: 3
Do you know the correct answer?
Acetylsalicylic acid (aspirin), HC9H7O4, is the most widely used pain reliever and fever reducer. Fi...
Questions in other subjects:

Spanish, 03.12.2021 14:00

Mathematics, 03.12.2021 14:00


Mathematics, 03.12.2021 14:00

Mathematics, 03.12.2021 14:00

Mathematics, 03.12.2021 14:00

English, 03.12.2021 14:00


Mathematics, 03.12.2021 14:00


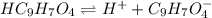
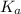 for above equation follows:
for above equation follows:![K_a=\frac{[C_9H_7O_4^-][H^+]}{[HC_9H_7O_4]}](/tpl/images/0515/8851/cb0ef.png)
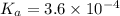
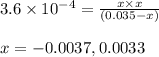
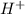 = x = 0.0033 M
= x = 0.0033 M![pH=-\log[H^+]](/tpl/images/0515/8851/cf945.png)