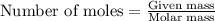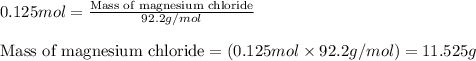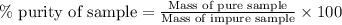Chemistry, 19.02.2020 05:58, jalenamaynard3989
A 12.00g sample of MgCl2 was dissolved in water. The solution was then treated with 0.2500mol of AgNO3 to precipitate all the chloride ions from the solution. Calculate the purity (as a mass percentage) of MgCl2 in the sample?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:00, hammackkatelyn60
The content of manganese (mn) in steel was determined spectrophotometrically and with the use of the standard addition method. an unknown sample of mn from a digested steel sample gave an absorbance of 0.185 when analyzed spectrophotometrically. when 5.00 ml of solution containing 95.5 ppm mn was added to 50.0 ml of the unknown steel solution (digested sample), the absorbance was 0.248. calculate the concentration, in parts-per-million (ppm), of mn in the digested steel sample solution.
Answers: 3

Chemistry, 22.06.2019 21:30, Lindsay882
While in europe, if you drive 125 km per day, how much money would you spend on gas in one week if gas costs 1.10 euros per liter and your car’s gas mileage is 32.0 mi/gal? assume that 1 euro=1.26 dollars
Answers: 2

Chemistry, 22.06.2019 22:30, brianna5626
How do limiting factors most affect population size? ostop population growthrestrict population growthincrease population sizeresult in positive impactso
Answers: 1
Do you know the correct answer?
A 12.00g sample of MgCl2 was dissolved in water. The solution was then treated with 0.2500mol of AgN...
Questions in other subjects:






History, 09.08.2021 23:50

Biology, 09.08.2021 23:50

History, 09.08.2021 23:50

Mathematics, 09.08.2021 23:50

Geography, 09.08.2021 23:50


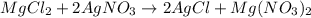
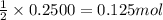 of magnesium chloride
of magnesium chloride