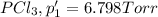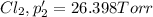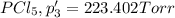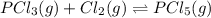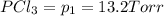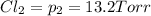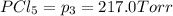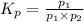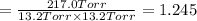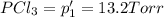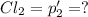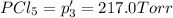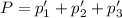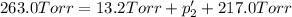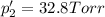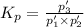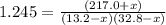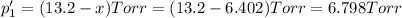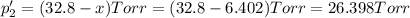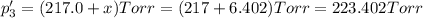An equilibrium mixture of PCl5(g), PCl3(g), and Cl2(g) has partial pressures of 217.0 Torr, 13.2 Torr, and 13.2 Torr, respectively. A quantity of Cl2(g) is injected into the mixture, and the total pressure jumps to 263.0 Torr (at the moment of mixing). The system then re-equilibrates. The appropriate chemical equation is PCl3(g)+Cl2(g) <-->PCl5(g) Calculate the new partial pressures after equilibrium is reestablished in torr

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, melidacampos12
Substance x has a fixed volume, and the attraction between its particles is strong .substance y had widely spread out particles and can be compressed what can most likely be concluded about these substances
Answers: 2

Chemistry, 22.06.2019 10:00, emfranco1
Ill give brainiestif one neutron initiates a fission event that produces two neutrons in the products, how many new reactions can now be initiated? if each of the neutrons produced in the first fission event then initiates a fission event that produces one neutron in the products, how many new reactions can now be initiated by each neutron? how many neutrons in total were produced by the two fission events described?
Answers: 2

Chemistry, 22.06.2019 14:40, neonbluefaith
Which statement best describes the function of enzymes?
Answers: 1
Do you know the correct answer?
An equilibrium mixture of PCl5(g), PCl3(g), and Cl2(g) has partial pressures of 217.0 Torr, 13.2 Tor...
Questions in other subjects:


History, 26.06.2019 12:30

Mathematics, 26.06.2019 12:30


History, 26.06.2019 12:30

Mathematics, 26.06.2019 12:30


Mathematics, 26.06.2019 12:30


Biology, 26.06.2019 12:30