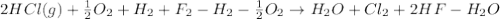Chemistry, 19.02.2020 02:23, Javanese5987
From the following enthalpies of reaction,
4 HCl (g) + O2 (g) → 2 H2O (l) + 2 Cl2 (g) ∆H = -202.4 kJ/mol 1/2 H2 (g) + ½ F2 (g) → HF (l) ∆H = -600.0 kJ/mol H2 (g) + ½ O2 (g) → H2O (l) ∆H = -285.8 kJ/mol
Calculate ∆Hrxn for 2 HCl (g) + F2 (g) → 2 HF (l) + Cl2 (g) Just input a number.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:00, natalie857123
For each of the following types of reactions, write a general reaction formula in the symbolic form—for example, a + b → ab. single-displacement double-displacement synthesis decomposition
Answers: 1



Chemistry, 23.06.2019 02:30, ggpro4life3000
Ascientist wants to know how individual lions within a pride interact with each other in their own environment. to do this, the scientist sedates and tags all of the lions within a pride. then, he places several remotely-controlled video cameras near the lions' den and performs an observational field study. he collects continuous video footage over the span of one year, analyzes the video, and then forms conclusions based on his observations.
Answers: 2
Do you know the correct answer?
From the following enthalpies of reaction,
4 HCl (g) + O2 (g) → 2 H2O (l) + 2 Cl2 (g) ∆H = -2...
4 HCl (g) + O2 (g) → 2 H2O (l) + 2 Cl2 (g) ∆H = -2...
Questions in other subjects:


Mathematics, 13.05.2021 22:00






Mathematics, 13.05.2021 22:00


Mathematics, 13.05.2021 22:00


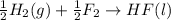 ΔH=-600.0 KJ/mol
ΔH=-600.0 KJ/mol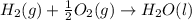 ΔH= -285.8 KJ/mol
ΔH= -285.8 KJ/mol