
Consider the following system at equilibrium: 2A(aq)+2B(aq)⇌5C(aq) Classify each of the following actions by whether it causes a leftward shift, a rightward shift, or no shift in the direction of the net reaction. a. increase (b)
b. increase(a)
c. increase(c)
d. decrease(a)
e. decrease(b)
f. decrease(c)
g. double(a) and reduce (b) to one half
h. double both (b) and (c)

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:40, loveoneonly9153
Consider this initial-rate data at a certain temperature for the reaction described by
Answers: 1

Chemistry, 22.06.2019 10:30, Riplilpeep
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1


Chemistry, 22.06.2019 20:00, SpiritedAway7087
In vapor-liquid equilibrium in a binary mixture, both components are generally present in both phases. how many degrees of freedom are there for such a system? the reaction between nitrogen and hydrogen to form ammonia occurs in the gas phase. how many degrees of freedom are there for this system? steam and coal react at high temperatures to form hydrogen, carbon monoxide, carbon dioxide, and methane. the following reactions have been suggested as being involved in the chemical transformation:
Answers: 3
Do you know the correct answer?
Consider the following system at equilibrium: 2A(aq)+2B(aq)⇌5C(aq) Classify each of the following ac...
Questions in other subjects:

History, 16.10.2020 14:01







English, 16.10.2020 14:01



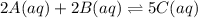
![K_{c}=\frac{[C(aq)]^5}{[A(aq)]^2\cdot [B(aq)]^2}](/tpl/images/0515/1184/e02e8.png)
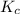 is the same, meaning that an increase in the concentration of the species B must cause a rightward shift to increase the concentration of the species C, such that the ratio expressed by the equilibrium constant remains unchanged.
is the same, meaning that an increase in the concentration of the species B must cause a rightward shift to increase the concentration of the species C, such that the ratio expressed by the equilibrium constant remains unchanged.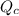 to compare with the equilibrium constant
to compare with the equilibrium constant ![Q{c}=\frac{[C(aq)]^5}{[A(aq)]^2\cdot [B(aq)]^2}](/tpl/images/0515/1184/564e9.png)




