

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:30, Wookas8355
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 22.06.2019 14:50, jonmorton159
Consider the following multistep reaction: a b→ab(slow) a ab→a2b(fast)−−−−−−−−−−−−−−−−− 2a b→a2b(overall) based on this mechanism, determine the rate law for the overall reaction. express your answer in standard masteringchemistry format. for example, if the rate law is k[a]3[b]2 type k*[a]^3*[b]^2
Answers: 3

Chemistry, 23.06.2019 00:20, destromero
Which diagram represents the phase tha occurs after a solid melts?
Answers: 1

Chemistry, 23.06.2019 10:30, piratesfc02
When a wire with a current is placed in a magnetic field, electrical energy is transformed into mechanical energy select the best answer from the choices provided t f
Answers: 2
Do you know the correct answer?
Consider the decomposition of red mercury(II) oxide under standard state conditions. )H0 T SFE ڮ( H...
Questions in other subjects:

Mathematics, 04.06.2021 05:10

Mathematics, 04.06.2021 05:10



Mathematics, 04.06.2021 05:10




Mathematics, 04.06.2021 05:10

English, 04.06.2021 05:10


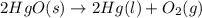
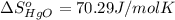
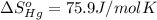
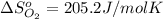
![\Delta S=[2\times \Delta S_{Hg}^o+1\times \Delta S_{O_2}^o]-[2\times \Delta S_{HgO}^o]](/tpl/images/0515/1320/fd534.png)
![=[2\times 75.9 J/mol K+1\times 205.2 J/molK]-[2\times 70.29 J/molK]=216.42 J/mol K](/tpl/images/0515/1320/fa874.png)




