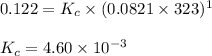Chemistry, 19.02.2020 01:49, mjessen119
Convert the values of Kc to values of Kp or the values of Kp to values of Kc.
A) N2(g)+3H2(g) <--> 2NH3(g); Kc=0.50 at 400 degrees Celsius.
B) H2+I2 <---> 2HI; Kc= 50.2 at 448 degrees Celsius.
C) Na2SO4*10H2O(s) <---> Na2SO4(s)+10H2O(g). Kp=4.08x10^-25 at 25 degrees Celsius.
D) H2O(l) <---> H2O (g); Kp= 0.122 at 50 degrees Celsius.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:50, suzymott1562
Nitrogen dioxide reacts with water to form nitric acid and nitrogen monoxide according to the equation: 3no2(g)+h2o(l)→2hno3(l)+no(g) part a suppose that 4.2 mol no2 and 0.50 mol h2o combine and react completely. which reactant is in excess? express your answer as a chemical formula. nothing
Answers: 1



Chemistry, 22.06.2019 16:50, lilblackbird4
Answer asap need by wednesday morning calculate the ph of 0.036m naoh best answer will be brainliest
Answers: 3
Do you know the correct answer?
Convert the values of Kc to values of Kp or the values of Kp to values of Kc.
A) N2(g)+3H2(g)...
A) N2(g)+3H2(g)...
Questions in other subjects:




Mathematics, 23.12.2020 23:40

English, 23.12.2020 23:40



Mathematics, 23.12.2020 23:40



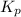 for the given equation is
for the given equation is 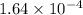
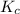 for the given equation is
for the given equation is 
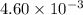
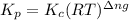 ..........(1)
..........(1)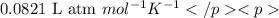
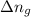 = change in number of moles of gas particles =
= change in number of moles of gas particles = 
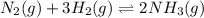
![K_c=0.50\\T=400^oC=[400+273]K=673K\\\Delta n_g=2-4=-2](/tpl/images/0515/1063/f4682.png)
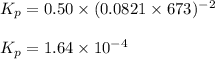
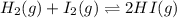
![K_c=50.2\\T=448^oC=[448+273]K=721K\\\Delta n_g=2-2=0](/tpl/images/0515/1063/bd740.png)
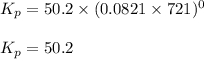
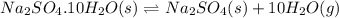
![K_p=4.08\times 10^{-25}\\T=25^oC=[25+273]K=298K\\\Delta n_g=10-0=10](/tpl/images/0515/1063/de076.png)
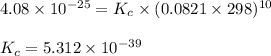
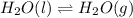
![K_p=0.122\\T=50^oC=[50+273]K=323K\\\Delta n_g=1-0=1](/tpl/images/0515/1063/26412.png)