Given the following at 25°C, calculate for HCN(g) (in kJ/mol) at 25°C.
; ΔH= –870.8 kJ
...


Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 16:30, ddmoorehouseov75lc
Correct relationship between molecular formula and empirical formula
Answers: 1

Chemistry, 22.06.2019 22:00, shaylasimonds587
The volume of an unknown substance in a sealed glass jar is 50 milliliters. the volume of the jar is 200 milliliters. which state of matter could the substance be?
Answers: 2

Chemistry, 23.06.2019 01:00, jaidencoolman2510
Na chemical reaction, activation energy increases the of the reactants. this outcome causes the particles to collide, which results in the of new products.
Answers: 2
Do you know the correct answer?
Questions in other subjects:


Mathematics, 24.08.2020 14:01



Arts, 24.08.2020 14:01

Mathematics, 24.08.2020 14:01


Mathematics, 24.08.2020 14:01


History, 24.08.2020 14:01


![\Delta H^o_{rxn}=\sum [n\times \Delta H_f_{(product)}]-\sum [n\times \Delta H_f_{(reactant)}]](/tpl/images/0514/9325/e893d.png)
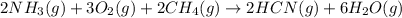
![\Delta H_{rxn}=[(2\times \Delta H_f_{(HCN(g))})+(6\times \Delta H_f_{(H_2O(g))})]-[(2\times \Delta H_f_{(NH_3(g))})+(3\times \Delta H_f_{(O_2(g))})+(2\times \Delta H_f_{(CH_4(g))})]](/tpl/images/0514/9325/ad92a.png)
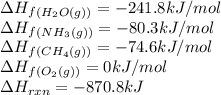
![-870.8=[(2\times \Delta H_f_{(HCN(g))})+(6\times (-241.8))]-[(2\times (-80.3))+(3\times (0))+(2\times (-74.6))]\\\\\Delta H_f_{(HCN(g))}=135.1kJ/mol](/tpl/images/0514/9325/0b580.png)




