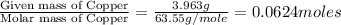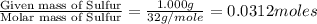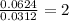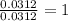Chemistry, 18.02.2020 23:54, Scoopaloop
In a chemical reaction, sulfur (S8) combines with copper to give a pure compound. If you start with 1.000 g of sulfur (S8) and obtain 4.963 g of pure compound. What is the empirical formula of this compound?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:40, deedee363
*will mark you brainliest + 15 points ** why does the equilibrium of a system shift when the pressure is increased? a. to maximize the stress on the system b. to stop restoring equilibrium to the system c. to increase the total moles of gas in the system d. to decrease the total moles of gas in the system
Answers: 3


Chemistry, 22.06.2019 12:30, murtaghliam1
Word equation for k(s) +h2o(l) yield koh (aq) + h2
Answers: 3

Chemistry, 22.06.2019 15:00, alanmarcus22
What does the symbol (–hfus) indicate in a phase change?
Answers: 1
Do you know the correct answer?
In a chemical reaction, sulfur (S8) combines with copper to give a pure compound. If you start with...
Questions in other subjects:




Social Studies, 18.05.2021 22:50

Social Studies, 18.05.2021 22:50



Mathematics, 18.05.2021 22:50