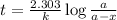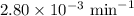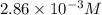G The gas phase decomposition of sulfuryl chloride at 600 K SO2Cl2(g) SO2(g) + Cl2(g) is first order in SO2Cl2 with a rate constant of 2.80×10-3 min-1. If the initial concentration of SO2Cl2 is 2.86×10-3 M, the concentration of SO2Cl2 will be M after 720 min have passed.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 18:10, bri9263
Consider the following reaction at equilibrium: c(s)+h2o(g)⇌co(g)+h2(g) predict whether the reaction will shift left, shift right, or remain unchanged upon each of the following disturbances. a) c is added to the reaction mixture. b) h2ois condensed and removed from the reaction mixture c) co is added to the reaction mixture d) h2 is removed from the reaction mixture.
Answers: 3

Chemistry, 22.06.2019 22:30, lanashanabJHsbd1099
Who discovered a pattern to the elements in 1869?
Answers: 1


Chemistry, 23.06.2019 06:30, lwattsstudent
When microscope slides are stained to show blood cells, the small red blood cells that appear on the slides are much numerous than the large white blood cells. this supports the concept that
Answers: 1
Do you know the correct answer?
G The gas phase decomposition of sulfuryl chloride at 600 K SO2Cl2(g) SO2(g) + Cl2(g) is first order...
Questions in other subjects:

Chemistry, 24.09.2021 14:00



English, 24.09.2021 14:00

History, 24.09.2021 14:00

English, 24.09.2021 14:00

Mathematics, 24.09.2021 14:00

English, 24.09.2021 14:00

Mathematics, 24.09.2021 14:00

Mathematics, 24.09.2021 14:00