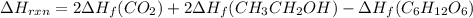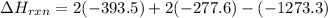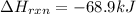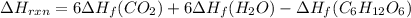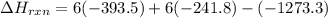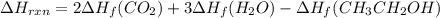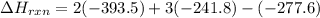Be sure to answer all parts. In winemaking, the sugars in grapes undergo fermentation by yeast to yield CH3CH2OH (ethanol) and CO2. During cellular respiration, sugar and ethanol are "burned" to water vapor and CO2.
a. Using C6H12O6 for sugar, calculate ΔH o rxn of fermentation and of respiration (combustion). Fermentation = kJ Respiration = kJ
b. Write a combustion reaction for ethanol. Include the physical states of each reactant and product. Which releases more heat from combustion per mole of C, sugar or ethanol? sugar ethanol

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 15:50, Edwardwall
Elements in group 2 are all called alkaline earth metals. what is most similar about the alkaline earth metals?
Answers: 1

Chemistry, 22.06.2019 16:00, bbrogle5154
If 15 drops of ethanol from a medical dropper weight 0.60g, how many drops does it takes from a dropper to dispense 1.0ml of ethanol? the density of ethanol is 0.80g/ml
Answers: 1

Chemistry, 22.06.2019 21:00, estherdinhllama
Read "who built the pyramids? ”. leave this link open while you answer the questions throughout the assignment. give at least two reasons why some people claim the pyramids of giza were constructed by aliens.
Answers: 1

Chemistry, 23.06.2019 04:30, Har13526574
Two liquids are poured into a beaker. after a few seconds, the beaker becomes warm. which of the following best describes this reaction? a. an exothermic reaction b. a decomposition reaction c. an endothermic reaction d. a single-displacement reaction
Answers: 1
Do you know the correct answer?
Be sure to answer all parts. In winemaking, the sugars in grapes undergo fermentation by yeast to yi...
Questions in other subjects:

Mathematics, 28.10.2019 08:31

Biology, 28.10.2019 08:31



Geography, 28.10.2019 08:31





Mathematics, 28.10.2019 08:31


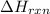 can be calculated using the standard enthalpy of formation (ΔHf) of products and reactants as follows:
can be calculated using the standard enthalpy of formation (ΔHf) of products and reactants as follows: 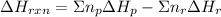 (1)
(1)