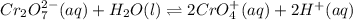Chemistry, 18.02.2020 21:48, Kennedy3449
Consider an acidic chromate-dichromate system at equilibrium in which the color is an orange-yellow. The reaction is represented by the equation:Cr2O72-(aq) + H2O(l)↔ CrO42+(aq) + H+(aq) orange yellow Which of the following best describes what happens if a strong base is added to the system at equilibrium? Explain your answer.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:00, hemolelekeakua
The alkali metals (group 1) consist of lithium (3), sodium (11), potassium (19), rubidium (37), cesium (55), and francium (87). they are soft, metallic solids with low densities and low melting points. based on the data shown in figure 1, how many valence electrons do alkali metals share?
Answers: 3

Chemistry, 22.06.2019 09:20, lanaiheart7
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2


Chemistry, 23.06.2019 07:50, alexusnicole817
Asolution is produced in which water is the solvent and there are four solutes. which of the solutes can dissolve better if the solution is heated?
Answers: 1
Do you know the correct answer?
Consider an acidic chromate-dichromate system at equilibrium in which the color is an orange-yellow....
Questions in other subjects:




English, 05.11.2020 21:10

Mathematics, 05.11.2020 21:10

English, 05.11.2020 21:10

Mathematics, 05.11.2020 21:10

Mathematics, 05.11.2020 21:10

Spanish, 05.11.2020 21:10

Mathematics, 05.11.2020 21:10