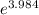The activation energy for the isomerization ol cyclopropane to propene is 274 kJ/mol. By what factor does the rate of this reaction increase as the temperature rises from 255 to 291 oC? Hint: The factor is the ratio of the rates. Since rate is directly proportional to rate constant, the factor is also ratio of rate constants.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:30, tntaylor862
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 1

Chemistry, 22.06.2019 09:20, payshencec21
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 23.06.2019 01:00, bsheepicornozj0gc
Reactions in cells take place at about a. 40°c b. 0° c. 100°c d. 60°c
Answers: 1

Chemistry, 23.06.2019 01:30, koggebless
The solubility of barium nitrate is 9.02 g/100 g h2o at 20°c. a 15.2 g sample of barium nitrate is added to 200.0 g of water at 20°c. is the solution saturated, unsaturated, or supersaturated? a. unsaturated b. saturated c. supersaturated
Answers: 1
Do you know the correct answer?
The activation energy for the isomerization ol cyclopropane to propene is 274 kJ/mol. By what factor...
Questions in other subjects:


Arts, 19.05.2021 02:20


History, 19.05.2021 02:20


Biology, 19.05.2021 02:20

Biology, 19.05.2021 02:20

Mathematics, 19.05.2021 02:20

Mathematics, 19.05.2021 02:20

Mathematics, 19.05.2021 02:20


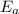 ) = 274 kJ/mol.
) = 274 kJ/mol. 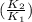 =
= ![\frac{E_a}{R} [\frac{1}{T_1}-\frac{1}{T_2}]](/tpl/images/0514/4438/3b4c3.png)
![\frac{273kJ/mol}{8.314*10^{-3}kJK^{-1}} [\frac{1}{528}-\frac{1}{564}]](/tpl/images/0514/4438/e9d63.png)
![\frac{273kJ/mol}{8.314*10^{-3}kJK^{-1}} [\frac{36k}{(528K)(564K)}]](/tpl/images/0514/4438/2ab49.png)