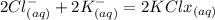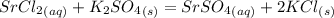When an aqueous solution of strontium chloride is added to an aqueous solution of potassium sulfate, a precipitation reaction occurs. Write the balanced net ionic equation of the reaction. Include charges on the ions, where applicable. Include coefficients only when they are different than ?

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 23.06.2019 01:00, zitterkoph
Which of the following is a physical change? a. burning a piece of wood b. sawing a piece of wood in half c. rust forming on an iron fence d. a copper roof changing color from orange to green
Answers: 1


Chemistry, 23.06.2019 04:20, milkshakegrande101
The equation below shows the reaction of zinc with hydrochloric acid (hcl). zn (s) + 2 hcl (aq) —> zncl2 (aq) + h2 (g) what will happen if the concentration of hcl is decreased? a. more zncl2 will be produced. b. the reaction rate will slow down. c. the hydrochloric acid will become more acidic. d. the reaction will produce water instead of hydrogen gas.
Answers: 1
Do you know the correct answer?
When an aqueous solution of strontium chloride is added to an aqueous solution of potassium sulfate,...
Questions in other subjects:

English, 13.12.2020 14:00

Biology, 13.12.2020 14:00


Arts, 13.12.2020 14:00

Arts, 13.12.2020 14:00


Mathematics, 13.12.2020 14:00

Mathematics, 13.12.2020 14:00


Advanced Placement (AP), 13.12.2020 14:00