Chemistry, 18.02.2020 18:35, adrian1742
The thermite reaction, used for welding iron, is the reaction of Fe3O4 with Al. 8 Al (s) + 3 Fe3O4 (s) ⟶ 4 Al2O3 (s) + 9 Fe (s) Δ H° = -3350. kJ/mol rxn. Because this large amount of heat cannot be rapidly dissipated to the surroundings, the reacting mass may reach temperatures near 3000. °C. How much heat (in kJ) is released by the reaction of 19.3 g of Al with 63.2 g of Fe3O4?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, pressure772
Which is a character of nuclear fusion but not nuclear fission
Answers: 3

Chemistry, 22.06.2019 09:00, 2024cynthiatercero
Which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 3

Do you know the correct answer?
The thermite reaction, used for welding iron, is the reaction of Fe3O4 with Al. 8 Al (s) + 3 Fe3O4 (...
Questions in other subjects:

Mathematics, 05.11.2020 02:10






Biology, 05.11.2020 02:10

Mathematics, 05.11.2020 02:20

English, 05.11.2020 02:20

Mathematics, 05.11.2020 02:20


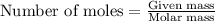
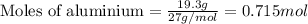
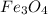 :
: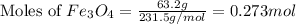
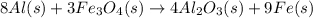
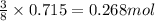 of
of 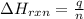
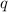 = amount of heat absorbed = ? J
= amount of heat absorbed = ? J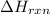 = enthalpy change of the reaction = -3350 kJ/mol
= enthalpy change of the reaction = -3350 kJ/mol