
The rate law for the reaction
2NO(g) + Cl2(g) → 2NOCl(g)
is given by R = k[NO][Cl2]
If the following is the mechanism for the reaction, NO(g) + Cl2(g) → NOCl2(g) NOCl2(g) + NO(g) → 2NOCl(g)
which of the following statements accurately describes this reaction? Check all that apply.
-2nd order reaction
-The first step is the slow step.
-Doubling [NO] would quadruple the rate.
-Cutting [Cl2] in half would decrease the rate by a factor of two.
-The molecularity of the first step is 1.
-Both steps are termolecular.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:30, MrSavannahCat
Clyde and marilyn are riding a roller coaster. during which section(s) of the track is their potential energy converted to kinetic energy? a. from point b to point c only b. from point b to point d only c. from point a to point b only d. from point a to point b and from point c to point d
Answers: 1



Chemistry, 23.06.2019 02:50, igraha17
Dumbledore decides to gives a surprise demonstration. he starts with a hydrate of na2co3 which has a mass of 4.31 g before heating. after he heats it he finds the mass of the anhydrous compound is found to be 3.22 g. he asks everyone in class to determine the integer x in the hydrate: na2co3·xh2o; you should do this also. round your answer to the nearest integ
Answers: 2
Do you know the correct answer?
The rate law for the reaction
2NO(g) + Cl2(g) → 2NOCl(g)
is given by R = k...
2NO(g) + Cl2(g) → 2NOCl(g)
is given by R = k...
Questions in other subjects:





History, 15.04.2020 01:59

Mathematics, 15.04.2020 01:59

Mathematics, 15.04.2020 01:59




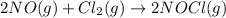
![k[NO][Cl_{2}]](/tpl/images/0514/1761/8cd0b.png)
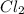 . Since, power of the concentrations of each of these is equal to 1. Therefore, order of the reaction is equal to 1 + 1 = 2.
. Since, power of the concentrations of each of these is equal to 1. Therefore, order of the reaction is equal to 1 + 1 = 2.



