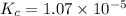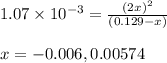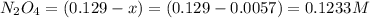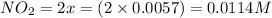Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:30, girlwholikesanime
Where are each of the three particles located within the atom?
Answers: 1

Chemistry, 22.06.2019 10:10, ragegamer334p3xlso
Stage in which a star’s outer layers have started to cool and grow outward?
Answers: 3


Chemistry, 22.06.2019 17:00, calmicaela12s
Which statement is true about a catalyst? a: a catalyst decreases the rate of the reaction. b. a catalyst is consumed during a chemical reaction. c. a catalyst lowers the activation energy of a reaction. d. a catalyst increases the reactant concentration during a reaction.
Answers: 1
Do you know the correct answer?
Assume that the change in concentration of N2O4 is small enough to be neglected in the following pro...
Questions in other subjects:

Mathematics, 08.06.2021 19:40


Business, 08.06.2021 19:40

Geography, 08.06.2021 19:40


Mathematics, 08.06.2021 19:40

Mathematics, 08.06.2021 19:40



History, 08.06.2021 19:40


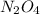 and
and 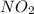 are 0.1233 M and 0.0114 M respectively
are 0.1233 M and 0.0114 M respectively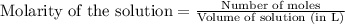
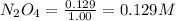
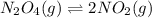
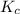 for above equation follows:
for above equation follows:![K_c=\frac{[NO_2]^2}{[N_2O_4]}](/tpl/images/0514/1717/271f5.png)