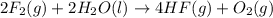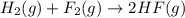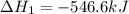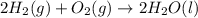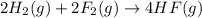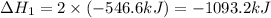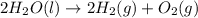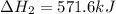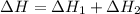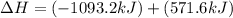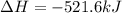Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 15:00, MilanPatel
How is the shape of the poem “peer” connected to its meaning?
Answers: 2

Chemistry, 22.06.2019 17:30, nijanicole164
A650 ml sodium bromine solution has a bromide ion concentration of 0.245 m. what is the mass (g) of sodium bromide in solution? a) 103.b)0.00155.c)16400.d) 16.4.e) 0.159
Answers: 2

Chemistry, 22.06.2019 21:30, Turtlelover05
How can the periodic table be used to predict the behavior of elements?
Answers: 1

Chemistry, 23.06.2019 07:50, dootdootkazoot
What is the significance sodium hydroxide and hydrochloric acid
Answers: 1
Do you know the correct answer?
Given that H2 (g) + F2 (g) ⟶ 2HF (g) ΔH ∘ rxn = − 546.6 kJ 2H2 (g) + O2 (g) ⟶ 2H2O (l) ΔH∘rxn = − 57...
Questions in other subjects:




Mathematics, 13.04.2021 15:30

Mathematics, 13.04.2021 15:30

History, 13.04.2021 15:40


Mathematics, 13.04.2021 15:40



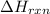 for the reaction is, -521.6 kJ
for the reaction is, -521.6 kJ