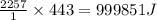Chemistry, 18.02.2020 06:27, rickyortega72701
As an athlete exercises, sweat is produced and evaporated to help maintain a proper body temperature. On average, an athlete loses approximately 443 g of sweat during an hour of exercise. How much energy is needed to evaporate the sweat that is produced? The heat of vaporization for water is 2257 J/g.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:50, justabeachbum
If a reactant was removed, did the new equilibrium system shift to make more reactants or more products?
Answers: 1


Do you know the correct answer?
As an athlete exercises, sweat is produced and evaporated to help maintain a proper body temperature...
Questions in other subjects:

Mathematics, 17.09.2020 06:01

Mathematics, 17.09.2020 06:01

Mathematics, 17.09.2020 06:01

Mathematics, 17.09.2020 06:01

Mathematics, 17.09.2020 06:01

Mathematics, 17.09.2020 06:01

English, 17.09.2020 06:01

Mathematics, 17.09.2020 06:01

Physics, 17.09.2020 06:01

Mathematics, 17.09.2020 06:01