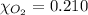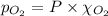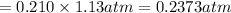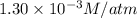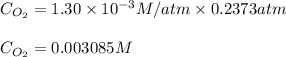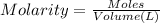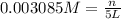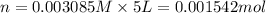Chemistry, 18.02.2020 03:26, netflixacc0107
Calculate the mass of oxygen gas (O2) dissolved in a 5.00 L bucket of water exposed to a pressure of 1.13 atm of air. Assume the mole fraction of oxygen in air to be 0.210 and the Henry's law constant for air in water at this temperature to be 1.30 × 10-3 M/atm. Report your answer in mg.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 22:40, destineysarah
Covalent bonds generally form when the bonded elements have a difference in electronegativity less than 1.5. subtract the electronegativities for the following pairs of elements and predict whether they form a covalent bond. electronegativity difference of c and c: ionic covalent electronegativity difference of mg and cl: ionic covalent
Answers: 1


Chemistry, 23.06.2019 01:30, Nakiahalogn4
How does the attraction between particles affect the ability of a solvent to dissolve in a substance
Answers: 1
Do you know the correct answer?
Calculate the mass of oxygen gas (O2) dissolved in a 5.00 L bucket of water exposed to a pressure of...
Questions in other subjects:



Mathematics, 26.07.2019 11:40


Mathematics, 26.07.2019 11:40


Mathematics, 26.07.2019 11:40

Mathematics, 26.07.2019 11:40



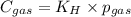
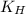 = Henry's constant =
= Henry's constant = 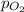 = partial pressure of oxygen
= partial pressure of oxygen