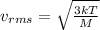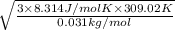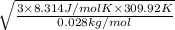Chemistry, 18.02.2020 01:01, yeidiyan9490
In a room full of air, the air is mainly composed of Nitrogen and Oxygen molecules (both at room temperature). Find (to two significant figures) the values of vrms for both molecules. (Eq. (20.26) relates vrms to absolute temperature.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:00, angtrevv
In a laboratory, 1.55mg of an organic compound containing carbon, hydrogen, and oxygen is burned for analysis. this combustion resulted in the formation of 1.45mg of carbon dioxide and .89 mg of water. what is the empirical formula for this compound?
Answers: 1

Chemistry, 22.06.2019 18:00, brisacruz013
Which statement best describes the he properties of iconic compounds ?
Answers: 1

Chemistry, 22.06.2019 19:00, miguel454545
Avolleyball player hit a ball with a mass of 0.25 kg. the average acceleration of the ball is 15.5 m/s². how much force did the volleyball player apply to the ball? 62.0 n 3.87 n 62.0 m/s² 3.87 m/s²
Answers: 2

Chemistry, 22.06.2019 19:20, choiboiqg5755
The equation picture below shows which type of nuclear reaction u 235 + n x e 134 + sr 100 + 2n
Answers: 1
Do you know the correct answer?
In a room full of air, the air is mainly composed of Nitrogen and Oxygen molecules (both at room tem...
Questions in other subjects:






History, 31.07.2019 01:00


Mathematics, 31.07.2019 01:00



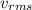 speed is as follows.
speed is as follows.