
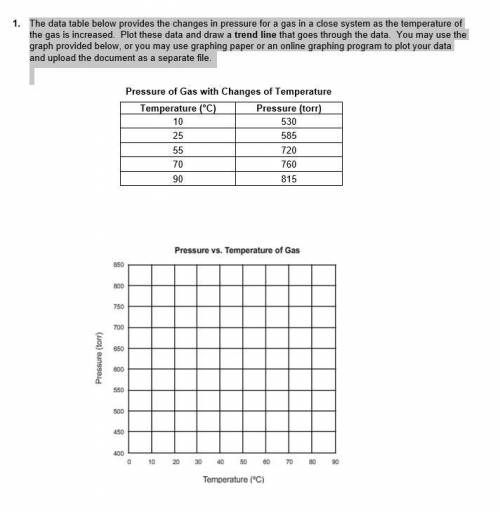
1. The data table below provides the changes in pressure for a gas in a close system as the temperature of the gas is increased. Plot these data and draw a trend line that goes through the data. You may use the graph provided below, or you may use graphing paper or an online graphing program to plot your data and upload the document as a separate file.
• Calculate the slope of the trend line including the units. Be sure to show all steps of your calculations.
• How are the variables of temperature and pressure related to one another?
• Use the trend line to determine what will be the gas pressure at 0°C.
i have attached a picture that shows the data tables

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:30, lpssprinklezlps
What method(s) do plants use to obtain nitrogen? select all that apply. absorb it from the atmosphere use bacteria to convert nitrogen to usable form obtain usable nitrogen compounds from the soil absorb nitrogen from water taken in at the roots
Answers: 3

Chemistry, 22.06.2019 14:30, davidrodriguez122001
Which of the following describes a situation where competition between producers exists
Answers: 1

Chemistry, 22.06.2019 23:00, edgar504xx
What is the solubility-product constant of barium sulfate, baso4, if a saturated solution is 1.03 ´ 10-5 m?
Answers: 3

Chemistry, 23.06.2019 01:00, ZaNiyahlove4711
Which of the following is the molecular formula for a simple sugar? a. cooh b. h2o c. oh d. c6h12o6
Answers: 1
Do you know the correct answer?
1. The data table below provides the changes in pressure for a gas in a close system as the temperat...
Questions in other subjects:


Mathematics, 10.12.2021 21:10

History, 10.12.2021 21:10


Biology, 10.12.2021 21:10

Mathematics, 10.12.2021 21:10


History, 10.12.2021 21:10

Mathematics, 10.12.2021 21:10






