
Chemistry, 17.02.2020 17:26, sedilei1515
Two equilibrium reactions of nitrogen with oxygen, with their corresponding equilibrium constants (Kc) at a certain temperature, are given below. reaction (1): N2(g) + O2(g) 2 NO(g); Kc = 1.54e-31 reaction (2): N2(g) + 1/2 O2(g) N2O(g); Kc = 2.61e-24 Using this set of data, determine the equilibrium constant for the following reaction, at the same temperature. reaction (3): N2O(g) + 1/2 O2(g) 2 NO(g)

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:50, sgslayerkingminecraf
Which of the following statements about acidic water is true? a. acid has no effect on the h, o molecules. b. the solution contains a larger number of oh ions than h, o ions. c. the solution contains a larger number of h, o ions than qh ions. d. the solution contains an equal number of h, o ions and oh ions. none of the above e.
Answers: 1

Chemistry, 22.06.2019 13:00, wbrandi118
Using the thermodynamic information in the aleks data tab, calculate the standard reaction free energy of the following chemical reaction: →+p4o10s6h2ol4h3po4s round your answer to zero decimal places.
Answers: 3


Chemistry, 22.06.2019 23:30, treylartigue
The appropriate concentration for an iodine sanitizer is
Answers: 1
Do you know the correct answer?
Two equilibrium reactions of nitrogen with oxygen, with their corresponding equilibrium constants (K...
Questions in other subjects:




Mathematics, 26.02.2020 16:46

Mathematics, 26.02.2020 16:46


Mathematics, 26.02.2020 16:46


World Languages, 26.02.2020 16:46


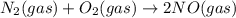 ; Kc = 1.54e - 31
; Kc = 1.54e - 31 ; Kc = 2.16e - 24
; Kc = 2.16e - 24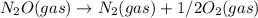 Kc = 1/2.16e - 24
Kc = 1/2.16e - 24 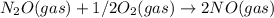 Kc = 1.54e-31 × 1/2.61e - 24
Kc = 1.54e-31 × 1/2.61e - 24




