
Chemistry, 17.02.2020 17:16, laurenlol756
During the rGFP purification experiment, the instructor will have to make breaking buffer for the students to use. This buffer contains 150mM NaCl. Given a bottle of crystalline NaCl (M. W. = 40g/mole), describe how you would make 500ml of 150mM NaCl. Include the type and sizes of any measuring vessels that you use to make the solution.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 21:40, fatherbamboo
A5 mole sample of liquid acetone is converted to a gas at 75.0°c. if 628 j are required to raise the temperature of the liquid to the boiling point, 15.600 kj are required to evaporate the liquid, and 712 j are required to raise the final temperature to 75.0°c, what is the total energy required for the conversion?
Answers: 3

Chemistry, 23.06.2019 03:30, uniqueray33
The molar mass of nickel(ni) is 58.7 g/mol. how many moles are in an 88 gram sample of nickel?
Answers: 1

Chemistry, 23.06.2019 05:40, Queenquestion9130
Why is any chemical reaction always balanced? give reasons and explain the easiest way to solve the balancing problems in chemical equations with stoichiometric coefficients upto 20 as hit and trial doesn't always work. give full reasoning
Answers: 1
Do you know the correct answer?
During the rGFP purification experiment, the instructor will have to make breaking buffer for the st...
Questions in other subjects:

Biology, 10.12.2020 18:10


Social Studies, 10.12.2020 18:10

Mathematics, 10.12.2020 18:10

Mathematics, 10.12.2020 18:10


History, 10.12.2020 18:10



Mathematics, 10.12.2020 18:10


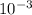 m
m × 500×
× 500×



