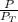A mixture of ethanol and 1−propanol behaves ideally at 36°C and is in equilibrium with its vapor. If the mole fraction of ethanol in the solution is 0.65, calculate its mole fraction in the vapor phase at this temperature. (The vapor pressures of pure ethanol and 1−propanol at 36°C are 108 and 40.0 mmHg, respectively.)

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:00, XxrazorxX11
How can you use chemical equations to predict the products of the reaction you can carry out?
Answers: 1

Chemistry, 22.06.2019 08:30, itzhari101
In a chemical reaction at equilibrium, the rate of the forward reaction the rate of the reverse reaction. if the rate of the forward reaction more products are formed.
Answers: 1

Chemistry, 23.06.2019 00:00, alisonsolis155
Before it was launched, a helium-filled balloon had a pressure of 201 kpa at a temperature of 27°c. at an altitude of 15,000 m, the pressure had decreased to 2.5 kpa and the temperature had dropped to -14 °c. the volume of the balloon increased to 59.3 m3. what is the original volume of the balloon? 13 m3 0.85 m3 0.077 m3 1.17 m3
Answers: 3
Do you know the correct answer?
A mixture of ethanol and 1−propanol behaves ideally at 36°C and is in equilibrium with its vapor. If...
Questions in other subjects:

English, 22.10.2020 01:01



History, 22.10.2020 01:01

History, 22.10.2020 01:01

English, 22.10.2020 01:01

Mathematics, 22.10.2020 01:01

Mathematics, 22.10.2020 01:01



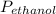 = 0.65 × 108 mmHg
= 0.65 × 108 mmHg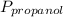 = 0.35× 40.0 mmHg
= 0.35× 40.0 mmHg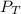 = 70.2 + 4.9 = 75.1 mmHg
= 70.2 + 4.9 = 75.1 mmHg