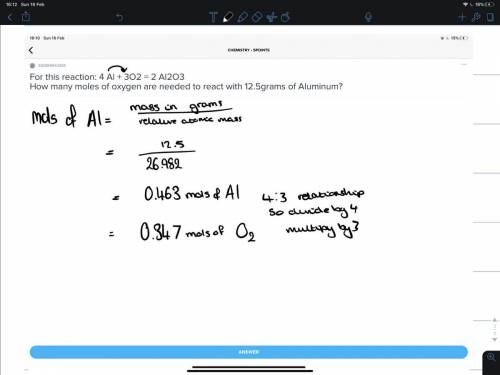
For this reaction: 4 Al + 3O2 = 2 Al2O3
How many moles of oxygen are needed to react with 12.5...

Chemistry, 16.02.2020 03:48, madeleine1102
For this reaction: 4 Al + 3O2 = 2 Al2O3
How many moles of oxygen are needed to react with 12.5grams of Aluminum?

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 03:30, ruleolivas
Asample of ammonia reacts with oxygen as shown. 4nh3(g) + 5o2(g) 4no(g) + 6h2o(g) what is the limiting reactant if 4.0 g of nh3 react with 8.0 g of oxygen? o2 because it produces only 0.20 mol of no. nh3 because it produces only 0.20 mol of no. o2 because it produces two times less no than nh3. nh3 because it produces three times more no than o2.
Answers: 3

Do you know the correct answer?
Questions in other subjects:

Arts, 20.03.2021 01:00


Mathematics, 20.03.2021 01:00


Arts, 20.03.2021 01:00

Mathematics, 20.03.2021 01:00

English, 20.03.2021 01:00


Mathematics, 20.03.2021 01:00