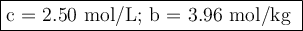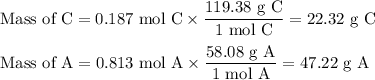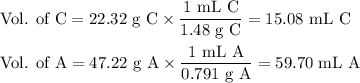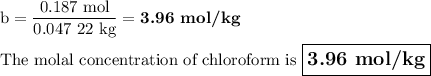Chemistry, 16.02.2020 02:36, keegandudley
A chemist combined chloroform (CHCl3) and acetone (C3H6O) to create a solution where the mole fraction of chloroform is 0.187. The densities of chloroform and acetone are 1.48 g/mL and 0.791 g/mL, respectively.
Calculate the molarity and molality of the solution.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 12:30, nestergurl101
Acycloalkane molecule contains 8 carbon atoms. how many hydrogen atoms are present in the molecule?
Answers: 2

Chemistry, 22.06.2019 15:20, merrickrittany
An alloy contains 66 g of pure zinc. what is the percentage of zinc in the alloy? express your answer to two significant figures and include the appropriate units.
Answers: 1

Chemistry, 22.06.2019 17:00, brandiwingard
What is the mass of phosphorous in a 51-kg person
Answers: 1

Do you know the correct answer?
A chemist combined chloroform (CHCl3) and acetone (C3H6O) to create a solution where the mole fracti...
Questions in other subjects:

Physics, 11.01.2020 16:31

History, 11.01.2020 16:31



Biology, 11.01.2020 16:31




Mathematics, 11.01.2020 16:31