
Chemistry, 15.02.2020 04:27, lekaje2375
A generic element, G, is composed of two isotopes, 132G and 128G. 132G has a natural abundance of 90% and an isotopic mass of 131.90 amu, and 128G has a natural abundance of 10% and an isotopic mass of 127.90 amu. What is the average atomic mass of this element?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:00, litttyyyu33411
Atrain travels 74 kilometers in 3 hours, and then 81 kilometers in 5 hours. what is its average speed?
Answers: 2

Chemistry, 22.06.2019 06:10, Kianna000
56.16 gregor mendel was the first scientist to use statistics to analyze scientific data. before mendel's experiments, scientists believed that organisms acquired traits from their environment and passed them on to their offspring. after mendel's discoveries were accepted, scientists realized that traits passed to offspring were the result of genes being passed from parents to offspring. this is an example of pls
Answers: 1

Do you know the correct answer?
A generic element, G, is composed of two isotopes, 132G and 128G. 132G has a natural abundance of 90...
Questions in other subjects:


Computers and Technology, 19.08.2021 21:00

English, 19.08.2021 21:00



Mathematics, 19.08.2021 21:00


Health, 19.08.2021 21:00



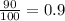
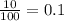
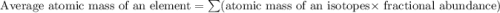
![A=\sum[(131.90\times 0.9)+(127.90\times 0.1)]=131.5amu](/tpl/images/0512/2891/2177c.png)





