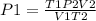Chemistry, 15.02.2020 04:22, carnations
A gas that has a volume of 28 L, a temperature of 45 °C, and an unknown pressure has its volume increased to 34 L and its temperature decreased to 35 °C. If the pressure is measured after the change to be 2.0 atm, what was the original pressure of the gas?A.1.6 atmB.2.5 atmC.3.2 atmD.4.1 atm

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:00, parisaidan366
What happened in 2012 and how does it illustrate the importance of understanding the sun and how it works?
Answers: 3

Chemistry, 22.06.2019 14:50, alexabbarker9781
How are evaporation and sublimation similar? a both involve the formation of a gas. b both release energy to the surroundings. c both take place throughout a solid. d both take place at the surface of a liquid.
Answers: 1

Chemistry, 22.06.2019 16:30, jrfranckowiak
At 20°c, a sample of h2o liquid and a sample of co2 gas each have the same average kinetic energy. why is one a liquid and the other a gas at this temperature?
Answers: 1

Chemistry, 22.06.2019 17:20, holmesleauja
Which of these features are formed when hot groundwater is forced out through cracks in the earth's surface?
Answers: 2
Do you know the correct answer?
A gas that has a volume of 28 L, a temperature of 45 °C, and an unknown pressure has its volume incr...
Questions in other subjects:




Mathematics, 11.02.2021 18:40

History, 11.02.2021 18:40

History, 11.02.2021 18:40


Mathematics, 11.02.2021 18:40