
Chemistry, 15.02.2020 04:27, emilymariec4036
The electron geometry and the molecular geometry of ammonia (NH3) are, respectively: The electron geometry and the molecular geometry of ammonia (NH3) are, respectively: tetrahedral, trigonal pyramidal. tetrahedral, tetrahedral. trigonal planar, bent. tetrahedral, bent. none of the above g

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:30, davidrodriguez122001
Which of the following describes a situation where competition between producers exists
Answers: 1

Chemistry, 22.06.2019 15:30, 20cschultz
Which suspect most likely committed the robbery and how do you know
Answers: 2

Chemistry, 23.06.2019 10:30, GiuliAzevedo
The element chlorine has two stable isotopes, chlorine-35 with a mass of 34.97 amu and chlorine-37 with a mass of 36.95 amu. from the atomic weight of cl = 35.45 one can conclude that:
Answers: 2
Do you know the correct answer?
The electron geometry and the molecular geometry of ammonia (NH3) are, respectively: The electron ge...
Questions in other subjects:



History, 09.09.2019 21:30


Biology, 09.09.2019 21:30

Mathematics, 09.09.2019 21:30



History, 09.09.2019 21:30


![\frac{1}{2}[V+N-C+A]](/tpl/images/0512/2895/0d710.png)
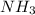
![\frac{1}{2}[5+3-0+0]=4](/tpl/images/0512/2895/ebbe0.png)
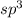 but as only 3 atoms are present , one position will be occupied by a lone pair thus electronic geometry is tetrahedral and the molecular geometry will be trigonal pyramidal.
but as only 3 atoms are present , one position will be occupied by a lone pair thus electronic geometry is tetrahedral and the molecular geometry will be trigonal pyramidal.



