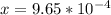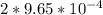Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 19:10, krisandlance
Astudent completes a titration by adding 12.0 milliliters of naoh(aq) of unknown concentration to 16.0 milliliters of 0.15 m hcl(aq). what is the molar concentration of the naoh(aq)? 1)5.0 m 2)0.20 m 3)0.11 m 4)1.1 m
Answers: 1

Chemistry, 23.06.2019 07:50, alexusnicole817
Asolution is produced in which water is the solvent and there are four solutes. which of the solutes can dissolve better if the solution is heated?
Answers: 1
Do you know the correct answer?
Hydrogen sulfide decomposes according to the following reaction, for which Kc = 9.30 × 10−8 at 700°C...
Questions in other subjects:

Arts, 10.12.2020 03:30


Mathematics, 10.12.2020 03:30


Mathematics, 10.12.2020 03:30



Mathematics, 10.12.2020 03:30

Physics, 10.12.2020 03:30


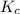 = 9.30 × 10⁻⁸
= 9.30 × 10⁻⁸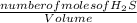
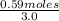
![K_c= \frac{[H_2]^2[S_2]}{[H_2S]^2}](/tpl/images/0511/9308/cf068.png)
![9.38*10^{-8}= \frac{[2x]^2[x]}{[0.1966-2x]^2}](/tpl/images/0511/9308/178bc.png)
![9.38*10^{-8}= \frac{[4x]^3}{[0.1966]^2}](/tpl/images/0511/9308/e52d1.png)
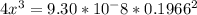
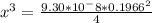
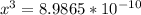
![x=\sqrt[3]{8.9865*10^{-10}}](/tpl/images/0511/9308/f6425.png)