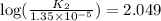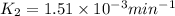Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:20, Naysa150724
If i can still dissolve more sugar into the solution at a certain temperature what would i call that solution
Answers: 3

Chemistry, 22.06.2019 07:20, rscvsdfsrysas1857
The diagrams show objects’ gravitational pull toward each other. which statement describes the relationship between diagram x and y? gravity attracts only larger objects toward one another. gravity attracts larger objects only if they are close to one another. if the masses of the objects increase, then the force between them also increases. if distance between the objects increases, then the amount of force also increases.
Answers: 1

Chemistry, 22.06.2019 14:40, neonbluefaith
Which statement best describes the function of enzymes?
Answers: 1

Chemistry, 22.06.2019 17:00, davisnaziyahovz5sk
The arrangement of particles is most ordered in a sample of
Answers: 1
Do you know the correct answer?
The gas phase reaction 2 N2O5(g) → 4 NO2(g) + O2(g) has an activation energy of 103 kJ/mol, and the...
Questions in other subjects:

English, 29.06.2019 23:00

Chemistry, 29.06.2019 23:00





Mathematics, 29.06.2019 23:00

Physics, 29.06.2019 23:00

Mathematics, 29.06.2019 23:00


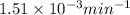
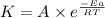
![\log (\frac{K_2}{K_1})=\frac{Ea}{2.303\times R}[\frac{1}{T_1}-\frac{1}{T_2}]](/tpl/images/0511/7907/6d953.png)
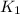 = rate constant at 266 K =
= rate constant at 266 K = 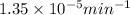
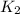 = rate constant at 296 K = ?
= rate constant at 296 K = ?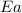 = activation energy for the reaction = 103 kJ/mol = 103000 J/mol
= activation energy for the reaction = 103 kJ/mol = 103000 J/mol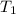 = initial temperature = 266 K
= initial temperature = 266 K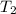 = final temperature = 296 K
= final temperature = 296 K![\log (\frac{K_2}{1.35\times 10^{-5}})=\frac{103000}{2.303\times 8.314J/mole.K}[\frac{1}{266}-\frac{1}{296}]](/tpl/images/0511/7907/d42c0.png)