
Reduction and oxidation must occur together. The electrons from the oxidized species are transferred to the reduced species. Chemists often break these two processes apart and write what are referred to as "half reactions." Consider these two half reactions: Zn2+ (aq) + 2 e- → Zn (s) Cu2+ (aq) + 2 e- à Cu (s) a. Are these oxidation or reduction half reactions? b. Calculate ΔG° for each of these reactions. Note that ΔG°f for the electron is 0 kJ/mol. c. Based on your answer to (b), does Zn2+ or Cu2+ more strongly favor reduction?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, saleenhernandez83
The climate of the continental united states is generally 1. tropical 2. temperate 3. arctic 4. highland
Answers: 1

Chemistry, 22.06.2019 08:30, dyanaycooper13
Analyze how limestone is weathered and identify the features that are formed as a result of this dissolution
Answers: 1


Chemistry, 22.06.2019 23:20, svaskeacevilles5477
In medium-sized stars such as the sun, nuclear fusion almost always means the fusing of nuclei to form , but larger stars can produce elements as heavy as
Answers: 2
Do you know the correct answer?
Reduction and oxidation must occur together. The electrons from the oxidized species are transferred...
Questions in other subjects:




Mathematics, 18.12.2020 01:00

Mathematics, 18.12.2020 01:00

Mathematics, 18.12.2020 01:00

Mathematics, 18.12.2020 01:00

Social Studies, 18.12.2020 01:00


Mathematics, 18.12.2020 01:00


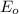 are as follows.
are as follows.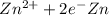 ,
, 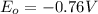
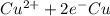 ,
, 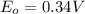
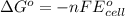
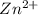 is as follows.
is as follows.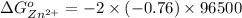
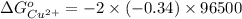
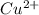 is the best oxidizing agent.
is the best oxidizing agent.




