
You double the partial pressure of a gas over a liquid at constant temperature. Which of these statements is then true? a. The Henry’s Law constant is decreased by half. b. There are half as many gas molecules in the liquid. c. There is no change in the number of gas molecules in the liquid. d. The Henry’s Law constant is doubled. e. There are twice as many gas molecules in the liquid.

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 23.06.2019 01:30, Sonicawesomeness
Select the correct answer from each drop-down menu. to make a table of the elements, dmitri mendeleev sorted the elements according to their . he then split the list of elements into several columns so that elements beside each other had similar .
Answers: 2

Chemistry, 23.06.2019 01:30, Nakiahalogn4
How does the attraction between particles affect the ability of a solvent to dissolve in a substance
Answers: 1

Chemistry, 23.06.2019 07:30, isalih7256
In the diagram here that represents the reaction, which reactant, a or b, is the limiting reagent?
Answers: 1
Do you know the correct answer?
You double the partial pressure of a gas over a liquid at constant temperature. Which of these state...
Questions in other subjects:



Mathematics, 28.02.2021 08:30

Chemistry, 28.02.2021 08:30

Mathematics, 28.02.2021 08:30


Computers and Technology, 28.02.2021 08:30

Mathematics, 28.02.2021 08:30

Mathematics, 28.02.2021 08:30


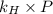
 = Henry's constant
= Henry's constant



