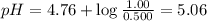Chemistry, 14.02.2020 03:28, andydiaz1227
What is the pH of a solution that is 0.500 M in acetic acid and 1.00 M in CH3COONa? Ka = 1.75*10-5 Group of answer choices 4.47 5.06 4.77 0.3 Flag this Question

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, palomaresmitchelle
There are 6.022, 104 atoms of hg in 1 mole of hg the number of atoms in 45 moles of hg can be found by multiplying 4.5 by 6.022, 102 which is the number of atoms in 4.5 moles of hg, correctly written in scientific notation with the correct number of significant figures? 0 21,109 0 21,100 271, 1024 27.099, 100 mark this and retum save and exit submit
Answers: 1


Chemistry, 22.06.2019 18:10, sangamlama
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1

Chemistry, 22.06.2019 19:30, Karinaccccc
How might this scientific phenomena be explained? a paper clip floats on water.
Answers: 1
Do you know the correct answer?
What is the pH of a solution that is 0.500 M in acetic acid and 1.00 M in CH3COONa? Ka = 1.75*10-5 G...
Questions in other subjects:




Mathematics, 05.09.2019 21:30

Geography, 05.09.2019 21:30

Mathematics, 05.09.2019 21:30


Biology, 05.09.2019 21:30

History, 05.09.2019 21:30