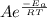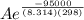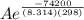The standard free energy of activation of one reaction A is 95.00 kJ mol–1 (22.71 kcal mol–1). The standard free energy of activation of another reaction B is 74.20 kJ mol–1 (17.73 kcal mol–1). Assume a temperature of 298 K and 1 M concentration.
By what factor is one reaction faster than the other?
Which reaction is faster?
(a) Reaction A is faster.
(b) Reaction B is faster.
(c) Cannot be determined.

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 23:30, sweaversw9602
What are the similarities between compounds and mixtures?
Answers: 3

Chemistry, 23.06.2019 05:30, brianrodriguez2005
What is the body’s main processing system? it uses input from various parts to control voluntary and involutiontary movement. it’s composed of two main parts-the brain and spinal cord. a. nbs b. cns c. ans d. pns
Answers: 1

Chemistry, 23.06.2019 14:00, wendyyy1214
If you fill your car tire to a pressure of 32 psi (pounds per square inch) on a hot summer day when the temperature is 35°c (95°f), what is the pressure (in psi) on a cold winter day when the temperature is -15°c (5°f)? assume no gas leaks out between measurements and the volume of the tire does not change.
Answers: 1
Do you know the correct answer?
The standard free energy of activation of one reaction A is 95.00 kJ mol–1 (22.71 kcal mol–1). The s...
Questions in other subjects:



Chemistry, 04.11.2020 22:50



Mathematics, 04.11.2020 22:50

Mathematics, 04.11.2020 22:50


Mathematics, 04.11.2020 22:50

Spanish, 04.11.2020 22:50