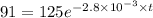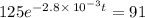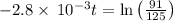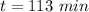The decomposition of dinitrogen pentoxide, N2O5, to NO2 and O2 is a first-order reaction. At 60°C, the rate constant is 2.8 × 10-3min-1. If a rigid vessel initially contains only N2O5 at a pressure of 125 kPa, how long will it take for the total pressure to reach 176 kPa?
a. 113 min
b. 129 min
c. 42 min
d. 182 min
e. 62 min
f. 83 min

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 23.06.2019 03:00, sharondacarruth1656
Is it safe to take 450mg of diphenhydramine hydrochloride?
Answers: 1
Do you know the correct answer?
The decomposition of dinitrogen pentoxide, N2O5, to NO2 and O2 is a first-order reaction. At 60°C, t...
Questions in other subjects:

Biology, 21.05.2020 08:57




Mathematics, 21.05.2020 08:57

History, 21.05.2020 08:57



Spanish, 21.05.2020 08:57

Advanced Placement (AP), 21.05.2020 08:57


![[A_t]=[A_0]e^{-kt}](/tpl/images/0510/6499/1ef89.png)
![[A_t]](/tpl/images/0510/6499/5262c.png) is the concentration at time t
is the concentration at time t
![[A_0]](/tpl/images/0510/6499/9a686.png) is the initial concentration
is the initial concentration
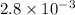 min⁻¹
min⁻¹