Chemistry, 13.02.2020 22:26, GreenHerbz206
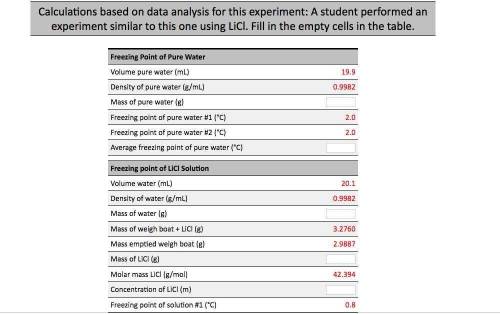
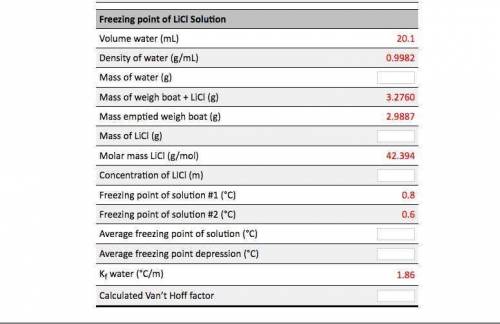
Calculations based on data analysis for this experiment: A student performed an experiment similar to this one using LiCl. Fill in the empty cells in the table. Freezing Point of Pure Water Volume pure water (mL) 19.9 Density of pure water (g/mL) 0.9982 Mass of pure water (8) Freezing point of pure water #1 (°C) Freezing point of pure water #2 (°C) Average freezing point of pure water (°C) Freezing point of Licl Solution 20.1 Volume water (mL) Density of water (g/mL) Mass of water (g) 0.9982 3.2760 Mass of weigh boat + LiCl (g) Mass emptied weigh boat (g) 2.9887 Mass of LiCl (8) 42.394 Molar mass LiCl (g/mol) Concentration of LiCl (m) Freezing point of solution #1 (°C) 0.8 Freezing point of Licl Solution Volume water (ml) 20.1 0.9982 3.2760 2.9887 = 42.394 Density of water (g/mL) Mass of water (g) Mass of weigh boat + LICI (g) Mass emptied weigh boat (g) Mass of LiCl (g) Molar mass Licl (g/mol) Concentration of LiCl (m) Freezing point of solution #1 (°C) Freezing point of solution #2 (°C) Average freezing point of solution (°C) Average freezing point depression (°C) Kf water (°C/m) Calculated Van't Hoff factor 0.8 1.86

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, dante766
Achemist 16 drop copper metal from copper chloride solution. the chemist place is 0.50 g of aluminum foil in a solution containing 0.75 g of copper (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction?
Answers: 1

Chemistry, 22.06.2019 17:30, tiffanyhmptn
Which scenario is most similar to the type of collision that gas particles have according to kinetic molecular theory
Answers: 1

Chemistry, 23.06.2019 03:50, timothymoles
Which best describes the activation energy of a chemical reaction? a. the combined energy of all the reactants b. the amount of energy required for a reaction to occur c. the difference in energy between products and reactants d. the potential energy stored in the bonds of reactants and products
Answers: 1
Do you know the correct answer?
Calculations based on data analysis for this experiment: A student performed an experiment similar t...
Questions in other subjects:





Mathematics, 16.04.2021 04:20


Biology, 16.04.2021 04:20

Mathematics, 16.04.2021 04:20