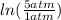Chemistry, 13.02.2020 22:22, haileyrae187
One mole of an ideal gas is contained in a cylinder with a movable piston. The temperature is constant at 778C. Weights are removed suddenly from the piston to give the following sequence of three pressures: a. P1 5 5.00 atm (initial state) b. P2 5 2.24 atm c. P3 5 1.00 atm (final state)

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:20, letsbestupidcx2314
Which of these conditions most likely produces an unstable isotope?
Answers: 1

Chemistry, 22.06.2019 12:30, poopybutt541
Avariable that is not being directly tested during an experiment should be
Answers: 1

Chemistry, 22.06.2019 18:00, rodriguezscarlet1713
Hydrogenation reactions, in which h2 and an "unsaturated" organic compound combine, are used in the food, fuel, and polymer industries. in the simplest case, ethene (c2h4) and h2 form ethane (c2h6). if 140 kj is given off per mole of c2h4 reacting, how much heat (in mj) is released when 12 kg of c2h6 forms?
Answers: 2

Chemistry, 22.06.2019 18:00, ambarpena14
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 2
Do you know the correct answer?
One mole of an ideal gas is contained in a cylinder with a movable piston. The temperature is consta...
Questions in other subjects:



Mathematics, 26.04.2020 01:35

Mathematics, 26.04.2020 01:35

English, 26.04.2020 01:35



History, 26.04.2020 01:35

Advanced Placement (AP), 26.04.2020 01:35


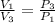
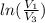 =
= 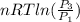
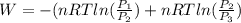 or
or 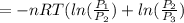
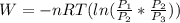 =
= 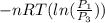 therefore the equation is the same for calculating directly from the initial pressure P₁, to the final pressure P₃
therefore the equation is the same for calculating directly from the initial pressure P₁, to the final pressure P₃