
Chemistry, 13.02.2020 22:08, DerekMoncoal
A student is given 1.4583 g of pure CuO. To recover the Cu present in the compound, the dark powdery solid was dissolved in 15.0 mL of 6 M HCl and the solution was diluted to 50.0 mL with water. How many grams of Mg is needed to displace all of the copper (II) ions from the solution? Mg(s) + Cu2+(aq) --> Cu(s) + Mg2+(aq)

Answers: 2
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 18:00, kyllow5644
Answer asap need to be answered by wednesday morning explain how a buffer works, using an ethanoic acid / sodium ethanoate system including how the system resists changes in ph upon addition of a small amount of base and upon addition of a small amount of acid respectively. include the following calculations in your i. calculate the ph of a solution made by mixing 25cm3 0.1m ch3cooh and 40cm3 0.1m ch3coo-na+. [ka = 1.74 x 10-5 m] ii. calculate the ph following the addition of a 10cm3 portion of 0.08 m naoh to 500cm3 of this buffer solution. iii. calculate the ph following the addition of a 10cm3 portion of 0.08 m hcl to 200cm3 of the original buffer solution.
Answers: 3

Chemistry, 22.06.2019 20:00, bettybales1986
Glucose (c6h12o6) is an important biological molecule. (round the answer to nearest hundredth.) what is the percent by mass of carbon in glucose?
Answers: 2
Do you know the correct answer?
A student is given 1.4583 g of pure CuO. To recover the Cu present in the compound, the dark powdery...
Questions in other subjects:


Spanish, 26.01.2021 03:00



Arts, 26.01.2021 03:00

Mathematics, 26.01.2021 03:00

Computers and Technology, 26.01.2021 03:00




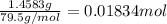
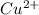 ions, then 0.01834 molesof CuO will have 0.01834 moles of
ions, then 0.01834 molesof CuO will have 0.01834 moles of 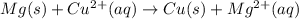
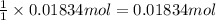 of magnesium.
of magnesium.



