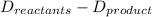Chemistry, 13.02.2020 20:53, camcollins00
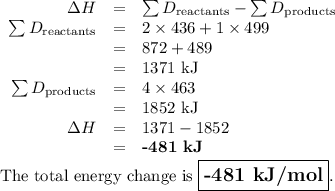
What is the total energy change for the following reaction: 2H2 + O2 -> 2H2O?
Given:
H-H bond: 436 kJ/mol
O-O double bond: 499 kJ/mol
H-O bond: 463 kJ/mol
A. -481 kJ/mol
B. + 445 kJ/mol
C. +63 kJ/mol
D. -730.5 kJ/mol

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:50, algahimnada
In a popular classroom demonstration, solid sodium is added to liquid water and reacts to produce hydrogen gas and aqueous sodium hydroxide. part a write a balanced chemical equation for this reaction. express your answer as a chemical equation. identify all of the phases in your answer.
Answers: 3

Chemistry, 21.06.2019 23:30, jescanarias22
What’s the scientific notation for the number 6,840,000,000
Answers: 1

Chemistry, 21.06.2019 23:50, kukisbae
How does a scientist the government? a. the scientist tells people in society what to do. b. the scientist determines the policies that the government spends money on. c. the scientist provides unbiased information to the government. d. the scientist makes laws based on his or her research results.
Answers: 1

Chemistry, 22.06.2019 14:30, Tooey2331
1) describe the physical layout of the ocean floor ? 2) explain how the dumbo octopus swims differently than other octopus species and why this would be an advantage in the aphonic zone . 3) why are the types of organisms that live at each underwater hot vent so dramatically different ?
Answers: 3
Do you know the correct answer?
What is the total energy change for the following reaction: 2H2 + O2 -> 2H2O?
Given:...
Given:...
Questions in other subjects:

Arts, 29.10.2020 08:10

Mathematics, 29.10.2020 08:10





Mathematics, 29.10.2020 08:10

Social Studies, 29.10.2020 08:10



 = ( A ) ; - 481 kJ/mol
= ( A ) ; - 481 kJ/mol