Chemistry, 13.02.2020 20:15, kkelley9223
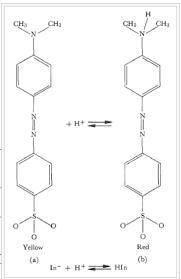
Methyl orange (HMO) is an acid-base indicator. Its two forms in solution are HMO (red) and MO- (yellow). When HMO is added to distilled water, the solution is yellow. How would you turn the solution red? a. add acid b. add base c. add distilled water

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, melidacampos12
Substance x has a fixed volume, and the attraction between its particles is strong .substance y had widely spread out particles and can be compressed what can most likely be concluded about these substances
Answers: 2


Do you know the correct answer?
Methyl orange (HMO) is an acid-base indicator. Its two forms in solution are HMO (red) and MO- (yell...
Questions in other subjects:





Computers and Technology, 13.01.2021 17:50

English, 13.01.2021 17:50

Biology, 13.01.2021 17:50