
Chemistry, 13.02.2020 19:57, CrusaderLord
For the reaction Fe3+(aq) + SCN-(aq) Fe(SCN)2+(aq). a. Addition of SCN-(aq) will shift the equilibrium in favor of products. b. Addition of SCN-(aq) will shift the equilibrium in favor of reactants. c. Addition of Cl-(aq) will shift the equilibrium in favor of reactants.

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 19:00, cindyroxana229
Nan element’s square on the periodic table, the number with the greatest numerical value represents the
Answers: 3

Chemistry, 23.06.2019 00:30, quintink
How can you write e method for the experiment of separating sand from water by filtration process? 1-materials 2-steps 3-conclusion also the same for the separating process of water and salt by filtration or distillation. quick because i need to finish my hw
Answers: 2
Do you know the correct answer?
For the reaction Fe3+(aq) + SCN-(aq) Fe(SCN)2+(aq). a. Addition of SCN-(aq) will shift the equilibri...
Questions in other subjects:

Mathematics, 13.02.2021 01:00

Mathematics, 13.02.2021 01:00

History, 13.02.2021 01:00




Mathematics, 13.02.2021 01:00




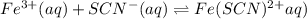
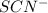 is increased on reactant side then the equilibrium will shift in the direction where decrease of concentration of
is increased on reactant side then the equilibrium will shift in the direction where decrease of concentration of 



