1. ClO-(aq) + H2O(l) => HClO(aq) + OH-(aq) SLOW

Chemistry, 13.02.2020 18:36, samantha9430
The proposed mechanism for a reaction is
1. ClO-(aq) + H2O(l) => HClO(aq) + OH-(aq) SLOW
2. I-(aq) + HClO(aq) => HIO(aq) + Cl-(aq) FAST
3. OH-(aq) + HIO(aq) => H2O(l) + IO-(aq) FAST
Which of the following would be a rate law for the reaction?
1. rate = k[ClO-][H2O][I-][OH-]
2. rate = k[ClO-][H2O]
3. rate = k[OH-][HIO]
4. rate = k[I-][HClO]
5. rate = k[ClO-][H2O][I-]

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 19:30, liyahlanderson2232
Astring vibrates with a frequency of 10 hz. why can't a person hear the sound waves produced by the vibrating string, no matter how large the amplitude of the waves? out! this is homework and due tomorrow! you so much!
Answers: 2


Chemistry, 23.06.2019 02:30, micahwilkerson9495
Which words or phrases identify layers of groundwater? check all that apply. water table kettle lake saturation zone underground lake sinkhole will give brainiest, answer quickly.
Answers: 1

Do you know the correct answer?
The proposed mechanism for a reaction is
1. ClO-(aq) + H2O(l) => HClO(aq) + OH-(aq) SLOW
1. ClO-(aq) + H2O(l) => HClO(aq) + OH-(aq) SLOW
Questions in other subjects:



Mathematics, 23.12.2021 14:00



Social Studies, 23.12.2021 14:00

English, 23.12.2021 14:00




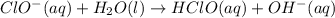 is a slow step reaction.
is a slow step reaction.![k[ClO^{-}][H_{2}O]](/tpl/images/0510/0437/87324.png)





