Chemistry, 13.02.2020 18:40, llamasking
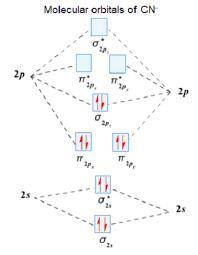
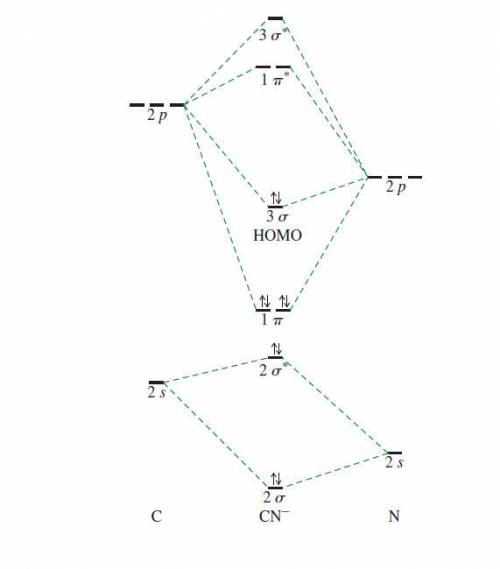
Using the molecular orbital model, write electron configurations for the following diatomic species and calculate the bond orders. Which ones are paramagnetic? Place the species in order of increasing bond length and bond energy.
a. CN +
b. CN
c. CN -

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 05:30, palcochran1313
Describe the interaction that occurs between two objects with the same electrical charge.
Answers: 1

Chemistry, 22.06.2019 13:30, bryce99
In a ni-cd battery, a fully charged cell is composed of nickelic hydroxide. nickel is an element that has multiple oxidation states. assume the following proportions of the states: nickel charge proportions found 0 0.17 +2 0.3 +3 0.33 +4 0.5 (a) determine the mean of the nickel charge. enter the answer to 2 decimal places.(b) determine the cumulative distribution function of nickel charge.
Answers: 2
Do you know the correct answer?
Using the molecular orbital model, write electron configurations for the following diatomic species...
Questions in other subjects:


Mathematics, 23.01.2021 01:00

History, 23.01.2021 01:00

Mathematics, 23.01.2021 01:00

English, 23.01.2021 01:00

Mathematics, 23.01.2021 01:00

Mathematics, 23.01.2021 01:00