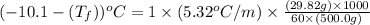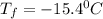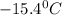A certain substance X has a normal freezing point of -10.1 degree C and a molal freezing point depression constant K_f = 5.32 degree C middot kg middot mol^-1. Calculate the freezing point of a solution made of 29.82 g of urea ((NH_2)_CO) dissolved in 500. g of X. Be sure your answer has the correct number of significant digits.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, ashleyjaslin
Which type of bonding involves the complete transfer of a valence electron from a less electrogrative atom to a more electronegative one
Answers: 1

Chemistry, 23.06.2019 12:00, Pointjazzyqueen602
How can nonpolar molecule contain polar covalent bonds
Answers: 1

Do you know the correct answer?
A certain substance X has a normal freezing point of -10.1 degree C and a molal freezing point depre...
Questions in other subjects:



Mathematics, 28.07.2019 19:10


Mathematics, 28.07.2019 19:10




Biology, 28.07.2019 19:10

Business, 28.07.2019 19:10


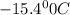
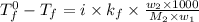
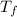 = boiling point of solution = ?
= boiling point of solution = ?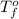 = boiling point of solvent (X) =
= boiling point of solvent (X) = 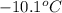
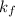 = freezing point constant =
= freezing point constant = 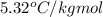
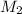 = molar mass of solute (urea) = 60 g/mol
= molar mass of solute (urea) = 60 g/mol