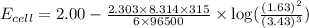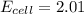Chemistry, 13.02.2020 05:32, Nicoleazaria
A galvanic cell at a temperature of 42 degrees Celcius is powered by the following redox reaction:
3CU2+(aq)+2Al(s)-->3Cu(s)+2Al3+( aq)
Suppose the cell is prepared with 3.43 M Cu2+n one half-cell and 1.63 M Al3+in the other.
Calculate the cell voltage under these conditions. Round your answer to 3 significant digits.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:20, jtingley0502
Temperature is _related to the average kinetic energy of a gas. inversely directly not disproportionally
Answers: 1

Chemistry, 22.06.2019 11:30, ashleybarrera2000
For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with ph. if the solubility does change, pick the ph at which you'd expect the highest solubility. you'll find ksp data in the aleks data tab. compounds does solubility change with ph
Answers: 3

Chemistry, 22.06.2019 14:00, ashlynneboogs0056
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 21:20, 50057543
Phosgene (carbonyl chloride), cocl2, is an extremely toxic gas that is used in manufacturing certain dyes and plastics. phosgene can be produced by reacting carbon monoxide and chlorine gas at high temperatures: co(g) cl2(g)⇌cocl2(g) carbon monoxide and chlorine gas are allowed to react in a sealed vessel at 477 ∘c . at equilibrium, the concentrations were measured and the following results obtained: gas partial pressure (atm) co 0.830 cl2 1.30 cocl2 0.220 what is the equilibrium constant, kp, of this reaction
Answers: 2
Do you know the correct answer?
A galvanic cell at a temperature of 42 degrees Celcius is powered by the following redox reaction:
Questions in other subjects:

Mathematics, 17.01.2021 23:00

Physics, 17.01.2021 23:00


Spanish, 17.01.2021 23:00


Computers and Technology, 17.01.2021 23:00


Business, 17.01.2021 23:00



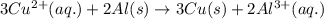
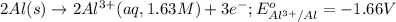 ( × 2)
( × 2)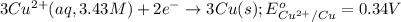 ( × 3)
( × 3)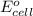 of the reaction, we use the equation:
of the reaction, we use the equation: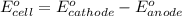
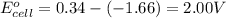
![E_{cell}=E^o_{cell}-\frac{2.303RT}{nF}\log \frac{[Al^{3+}]^2}{[Cu^{2+}]^3}](/tpl/images/0509/7294/3aff8.png)
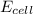 = electrode potential of the cell = ? V
= electrode potential of the cell = ? V![42^oC=[42+273]K=315K](/tpl/images/0509/7294/563a7.png)
![[Cu^{2+}]=3.43M](/tpl/images/0509/7294/455ea.png)
![[Al^{3+}]=1.63M](/tpl/images/0509/7294/1e5e1.png)