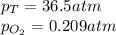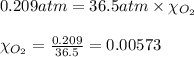Chemistry, 13.02.2020 05:14, jrassicworld4ever
What should be the mole fraction of O2 in the gas mixture the diver breathes in order to have the same partial pressure of oxygen in his lungs as he would at sea level? Note that the mole fraction of oxygen at sea level is 0.209.

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 21.06.2019 22:10, YatesDevon3371
Which form of relativism states that people rely on their own standards of right and wrong when making a decision?
Answers: 1

Chemistry, 22.06.2019 13:30, Sbeech7246
Why does asexual reproduction result in offspring with identicle genetic variation
Answers: 2

Chemistry, 22.06.2019 14:00, BrandyLeach01
How does the presence of oxygen affect the chemical pathways used to extract energy from glucose?
Answers: 3
Do you know the correct answer?
What should be the mole fraction of O2 in the gas mixture the diver breathes in order to have the sa...
Questions in other subjects:

Social Studies, 13.07.2019 05:30

History, 13.07.2019 05:30

Social Studies, 13.07.2019 05:30

Mathematics, 13.07.2019 05:30


Biology, 13.07.2019 05:30

Chemistry, 13.07.2019 05:30

Social Studies, 13.07.2019 05:30

History, 13.07.2019 05:30

Mathematics, 13.07.2019 05:30


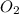 in the gas mixture the diver breathes in order to have the same partial pressure of oxygen in his lungs as he would at sea level? Note that the mole fraction of oxygen at sea level is 0.209.
in the gas mixture the diver breathes in order to have the same partial pressure of oxygen in his lungs as he would at sea level? Note that the mole fraction of oxygen at sea level is 0.209.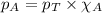 ........(1)
........(1)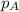 = partial pressure of oxygen at sea level = ?
= partial pressure of oxygen at sea level = ?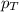 = total pressure at sea level = 1.00 atm
= total pressure at sea level = 1.00 atm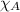 = mole fraction of oxygen at sea level = 0.209
= mole fraction of oxygen at sea level = 0.209